


Some of the transition metals' visible spectra are unique looking. We've already seen copper, silver, zinc, etc. which are very recognizable. Here are a few that are almost as distinctive.
  |
  |
  |
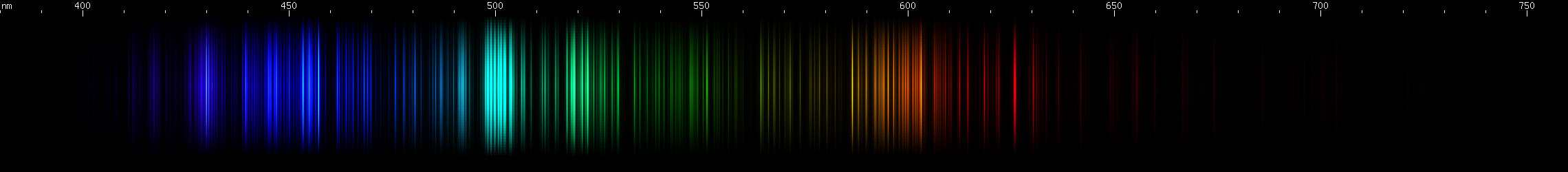
Titanium: By far its brightest feature visually is the tight cluster of many teal lines near 500nm, accompanied by emerald and grass-green brightnesses. A weaker cluster occurs in the yellow-orange regions. In the blue-violet, an indigo stripe (~440-460nm) is followed by bright indigo-violet (430nm) and then two more violet.
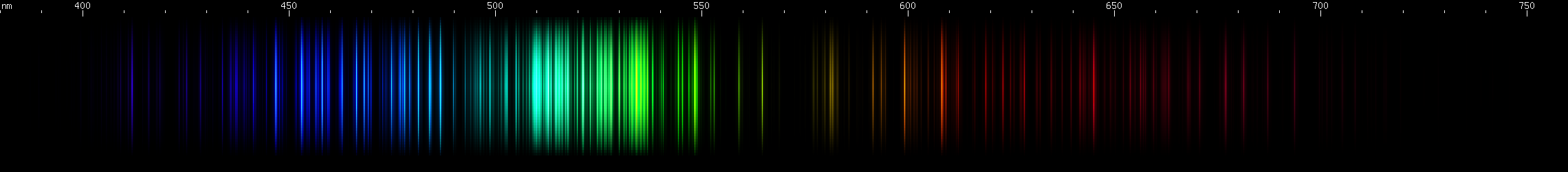
Cobalt: The green region is lit up by several bright peaks between 510nm and almost 540nm. Some yellow-green occurs nearby, and a chartreuse line further out. Past that is a bit of orange, an orange-red line, and then a deep red line some distance out. To shorter wavelengths, three discrete bright azure lines are followed by a hazier bright area and then one more line. Then several blue lines culminating in two strong indigo lines. Deeper into the violet region is a single intense line.
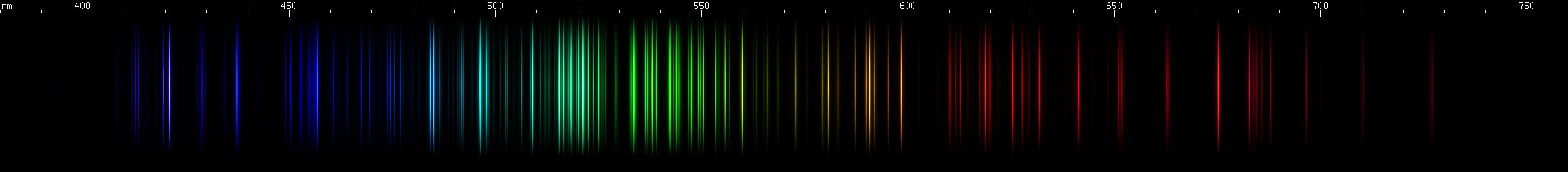
Rhodium: The most expensive stable metal doesn't disappoint. After some moderately weak red lines, there's a pattern of sharp orange, dimmer amber, and diffuse yellow. A single strong chartreuse line begins a sequence of green featuring two bright stripes of grass-green and emerald. Off the edge of the emerald stripe is a weaker teal line, then a stronger teal and an azure. After some faint to moderate indigo lines come four evenly spaced intense violet lines, the third one strongest.
Some of the transition metals resemble other transition metals, or other elements entirely.
  |
  |
 |
  |
  |
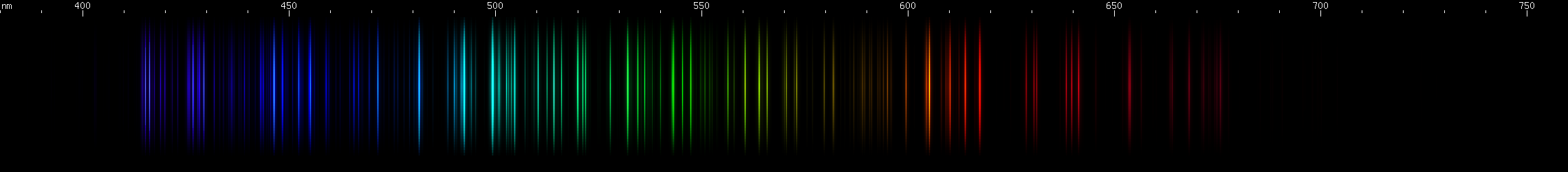  |
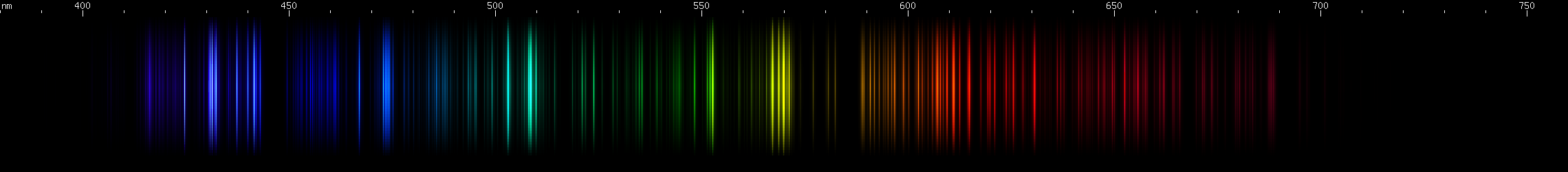
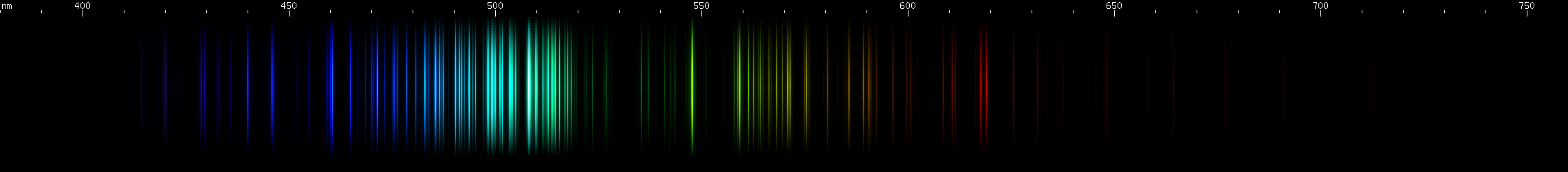
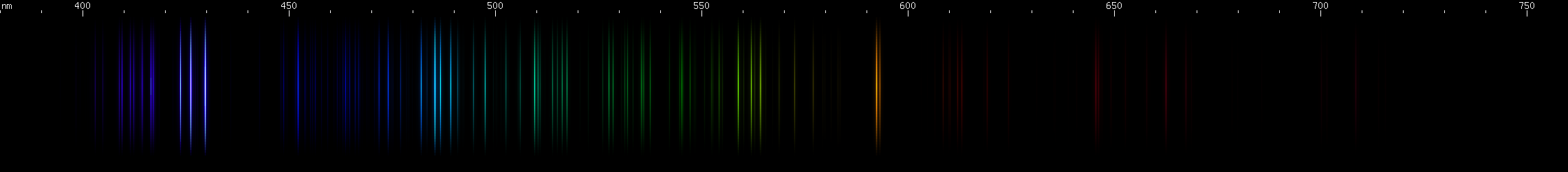
Scandium vs. Vanadium: Scandium's bright greenish-yellows are in a narrow strip, compared to vanadium's wide 560-585nm swath. In the greens and blues, scandium has three distinct, evenly spaced bright regions in teal, blue, and violet, while vanadium has a more aqua brightness and several indigo-violet lines in a distinctive pattern. Scandium shows a narrow group of orange-red lines, while vanadium has a wider red swath followed by a deeper red and then a fainter cherry red.
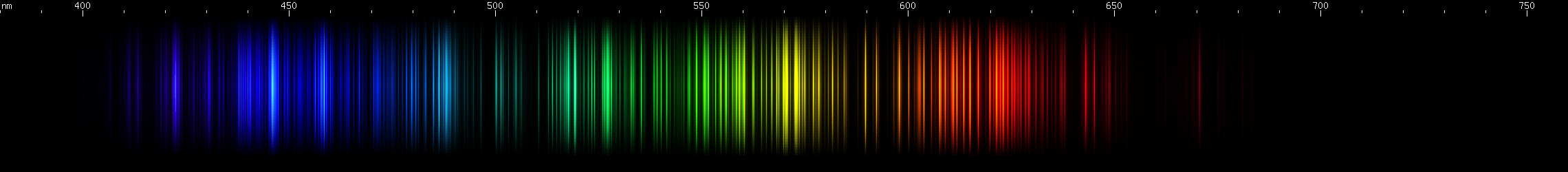
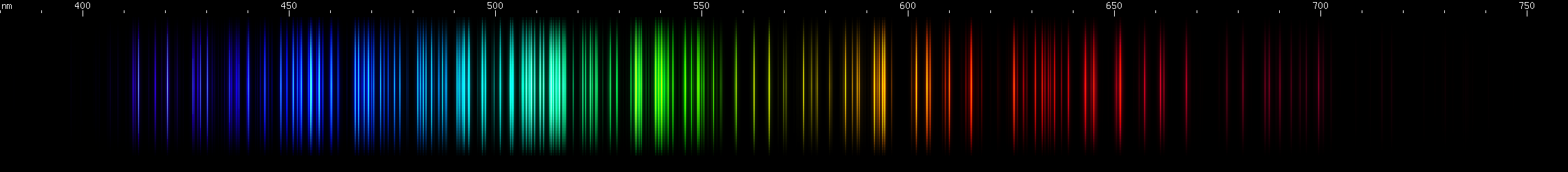
Nickel vs. Tantalum: Under certain conditions these two metals can be lookalikes; tantalum has a teal stripe of bright lines that resembles nickel. Nickel's visible spectrum is overwhelmingly dominated by its large number of bright blue-green lines, peaking in the teal region and trailing off into hues of blue. Tantalum's blue-green lines are much less prominent and occur in a confined region with well defined edges. Nickel also has a single bright grass-green line and then a sort of block of lines running from chartreuse to yellow, brightest at the two ends. That combined with its discrete orange line and red line, makes a pattern that is unmistakably nickel. Tantalum's green lines are more uniformly bright, with no single line that stands out. Its yellows and reds are also more numerous and more uniform than nickel's. In the blue region, nickel again gives a distinctive pattern, this time alternating dim and bright lines where the cyan showiness trails off, and then two more bright indigo lines and some violet. Tantalum is stronger in violet lines than blue lines, with a few moderately bright blue stripes instead of having any discrete blue lines.
Technetium vs. Sulfur: I have not procured any technetium to take a photo, although it is available but in a quantity that would probably not be sufficient. The sulfur photo is from a spectrum tube after digitally removing argon lines as much as possible. The two auto-generated spectra, however, show striking similarities except for sulfur's lack of a strong amber line. The best way to distinguish them might be to look at the pattern of lines in the cyan through violet regions, although sulfur's two triplets near 630nm and 640nm are, in the source data, supposedly brighter than the photo indicates.
Next: Yttrium and The Lanthanides